Scroll to:
The influence of adiponectin on carbohydrates, lipids, and lipoproteins metabolism: analysis of signaling mechanisms
https://doi.org/10.14341/omet12754
Abstract
Dysregulation of adipose tissue functions makes a significant contribution to the pathogenesis of metabolic syndrome, one of the most common diseases in recent years. Adipose tissue is an organ that secretes at least several dozen signaling molecules, adipokines. One of the most studied and at the same time mysterious adipokines is adiponectin. The latter is due to the lack of clear ideas about the biological role of this adipokine, the presence of its several molecular forms with different activity and several types of receptors to this adipokine localized in almost all cells of the body. The purpose of this review is to summarize and analyze the available information about the molecular mechanisms of the effect of adiponectin on metabolism of carbohydrates, lipids and lipoproteins. The literature search was conducted by the keywords "adiponectin" and "metabolic syndrome" in the Pubmed and Elibrary.ru databases for the period from 1995 to 2021.
According to the results of the literature analysis, it is assumed that adiponectin is involved in energy metabolism as a «satiety» hormone that promotes the utilization and storage of energy-rich substrates, fatty acids and glucose, which prevents the development or mitigates the already developed insulin resistance. This reduces the amount of plasma triglycerides and increases the level of high-density lipoproteins in the plasma. Adiponectin affects metabolic processes by activating the AdipoR1-APPL1-LKB1-AMPK, AdipoR1-APPL1-p38, AdipoR2-PPARa cascades, and possibly by activating the ceramidase and phosphoinositide pathways and insulin signaling. In addition to the AdipoR1/2 receptors, the adhesion molecule T-cadherin may be involved in the transduction of the adiponectin signal in endothelial and muscle cells. The mechanisms of signal transduction from T-cadherin, as well as from AdipoR2, remain unclear. Studies on the mechanisms of the action of individual molecular forms of adiponectin are extremely rare. The analysis shows the complex nature of adiponectin signaling, many of the mechanisms of which remain undiscovered, and it is possible that the near future will bring us significant progress in this area.
Keywords
For citations:
Tanyanskiy D.A., Denisenko A.D. The influence of adiponectin on carbohydrates, lipids, and lipoproteins metabolism: analysis of signaling mechanisms. Obesity and metabolism. 2021;18(2):103-111. https://doi.org/10.14341/omet12754
INTRODUCTION
For a long time, adipose tissue was considered mainly as a storage of excess energy in the form of triglycerides (TG), as well as a tissue that insulates and mechanically supports internal organs. However, the discovery in 1994 of leptin, a «satiety factor» produced primarily by adipocytes, revealed another function of adipose tissue. It has been found that this tissue secretes signals that regulate food intake and energy expenditure, thus coordinating changes in energy balance and nutritional status throughout the body. Later, many factors secreted by adipose tissue were discovered, which made it possible to consider it as an endocrine organ. Some of these factors can directly stimulate the development of adipose tissue, ensuring the availability of healthy adipose tissue, capable of meeting all the requirements of the need for energy storage arising from a positive energy balance [1].
Among many regulatory molecules secreted by adipose tissue, adiponectin, discovered in the mid-90s of the last century, probably attracts the greatest attention of researchers [2]. This is due not only to the fact that, unlike most adipokines, the production of adiponectin in obesity is decreasing, but also to an extremely wide range of target tissues and biological effects of this protein. At the same time, the study of the physiological role of adiponectin and its biological effects was significantly complicated by the presence of several molecular forms of adiponectin with different biological activities and binding them to at least two types of receptors present on almost all cells of the body [3]. In this regard, deciphering the mechanisms of adiponectin signal transduction into the cell is very important both for understanding the effect of this protein on different types of tissues and for a possible therapeutic intervention in these effects.
The purpose of this review is to analyze current information on signaling pathways and molecular mechanisms of the effect of adiponectin on energy metabolism, i.e. on the metabolism of carbohydrates, lipids and lipoproteins (LP). The search for literature sources was carried out using the keywords «adiponectin» and «metabolic syndrome» in the Pubmed and Elibrary.ru databases for the period from 1995 to 2021.
MOLECULAR FORMS OF ADIPONECTIN
Adiponectin circulates in the blood in various molecular forms: trimers, hexamers, and multimers [4]. Each adiponectin monomer (~ 30 kDa) consists of four sequential regions: an N-terminal signal sequence, a variable fragment (non-homologous to other proteins), a collagen-like domain, and a C-terminal globular domain [2]. The globular adiponectin domain, constituting slightly more than half of the protein mass, can be split off during limited proteolysis; small amounts of this fragment are found in human plasma [5]. Apparently, the splitting off the globular domain of adiponectin occurs in tissues under the action of various proteases; the specific enzyme that cleaves adiponectin has not been found [6]. It has been shown that the globular form of adiponectin has biological activity [3][5]. However, whether this adiponectin form plays any role in the body remains unclear.
According to cryo-electron microscopy, trimers are structures in the shape of three heads formed by globular adiponectin domains located on a single stalk, consisting of triple helix of collagen-like domains. A couple of trimers, fastened parallel to each other, are involved in the construction of hexamers. In hexameric structure, trimers are oriented head-to-head resembling the letter «Y». Multimers are formed by twisting collagen-like domains of trimers and hexamers around a single rod. Thus, a “bouquet of buds” is formed, which is structurally similar to the complement factor C1q, mannan-binding lectin, and a number of other similar multimeric proteins [4].
Adiponectin multimerization occurs intracellularly with involvement of endoplasmic reticulum (ER) chaperones BiP, ERp44, foldase Ero1-Lα, and protein disulfide isomerase DsbA-L [7][8]; in the bloodstream, the interconversion of adiponectin molecular forms does not occur [9]. Point mutations in the adiponectin gene have been described, resulting in impaired ability to form multimeric structures of the protein [10][11].
Adiponectin oligomers and multimers have different affinity for receptors and, as a consequence, might have diverse biological effects [3][12-14]. In this regard, one of the ways to regulate both the adiponectin production and its biological effects is to change the degree of its multimerization by varying the concentration of chaperones in the cells [8][15].
Finally, it should be noted that the concentration of adiponectin in women typically is higher than in men, especially for its high-molecular forms: the content of adiponectin multimers in plasma is approximately 3 times higher in women than in men [10].
THE INFLUENCE OF ADIPONECTIN ON CARBOHYDRATE, LIPID, AND LIPOPROTEIN METABOLISM
Unlike other adipokines, adiponectin production by adipose tissue and its blood concentration in patients with obesity are decreasing [16], while adiponectin synthesis in differentiating adipocytes, on the contrary, is increasing [2]. Adiponectin enhances the differentiation of adipocytes by itself [17][18]. In this regard, a decrease in adiponectin production in obesity can be considered as a compensatory mechanism aimed at limiting the growth of adipose tissue. The mechanism of this feedback regulation remains unclear.
Adiponectin has a wide range of metabolic effects. Primarily, the action of adiponectin is aimed at regulating energy metabolism. Thus, along with the acceleration of the adipocytes maturation, this adipokine activates the uptake of glucose and fatty acids (FA) by those cells [17][19] and suppresses TG lipolysis and the release of FA from adipose tissue [20][21]. As a whole, these events lead to an increase in lipids deposition in adipocytes and expansion adipose tissue. In addition, adiponectin stimulates the synthesis and secretion of lipoprotein lipase by fat cells, an enzyme that breaks down TGs of plasma LP that also promotes FA uptake by adipocytes [22]. At the same time, adiponectin induces the uptake and degradation of fatty acids and glucose in muscles with simultaneous activation of the synthesis of protein, which uncouples oxidation and phosphorylation [5][23–25]. It allows the “burning” of energy-rich substrates without excessive accumulation of ATP and NADH, powerful allosteric inhibitors of the Krebs cycle. It is in a good agreement with the indicated effects of adiponectin and its ability to reduce insulin resistance (IR) [24][26][27], and then to an increase in the uptake and breakdown of glucose by muscles and adipose tissue (this is necessary for deposition of FA in the form of triglycerides, since adipose tissue practically does not capture glycerol from the blood).
Thus, adiponectin is a kind of a «satiety hormone» that promotes the utilization and storage of energy-rich substrates (FA and glucose), preventing development or alleviating the already developed IR. In addition, adiponectin has some insulin-like effects: it promotes the glucose uptake by muscles and adipose tissue using GLUT4 [17], suppresses gluconeogenesis in the liver [28] and lipolysis in adipose tissue [20, 21].
The adiponectin-activated capture of FA by adipose tissue and muscles, along with the suppression of their release from the adipose tissue, leads to a lowering the concentration of FA in blood and, consequently, to a decrease in their supply to the liver. This, in turn, slows down the synthesis and secretion of TG by the liver, leading to a decrease in the TG level in blood. This is also facilitated by the acceleration of TG breakdown in the bloodstream by lipoprotein lipase (Fig. 1).
The indicated biological effects of adiponectin are in a good agreement with numerous clinical observations. Thus, it was found that with a decrease in the blood content of adiponectin in obese individuals, its concentration negatively correlates with IR, plasma concentrations of FA, TG and positively - with the concentration of high-density LP cholesterol (HDL-C) in plasma [29]. The above parameters are related mainly with the content of multimeric but not oligomeric adiponectin forms in plasma [30].
Multiple regression analysis indicate that plasma concentrations of adiponectin and leptin are independent determinants of IR [31]. In addition, the adiponectin content was an independent determinant of the concentration of TG [32], HDL-C [33], or both lipid parameters [29]. The positive relationship between plasma concentrations of adiponectin and HDL-C may be due to a decrease in the plasma TG level by the former [22] leading to slowing down HDL catabolism [34]. Besides, adiponectin stimulates the synthesis of apolipoprotein (apo) A-1, the main protein of HDL, in the liver [22][35] (Fig. 1). Adiponectin suppresses hepatic secretion of apoB, which may contribute to a decrease in the plasma level of apoB-containing LP by the adipokine [35].
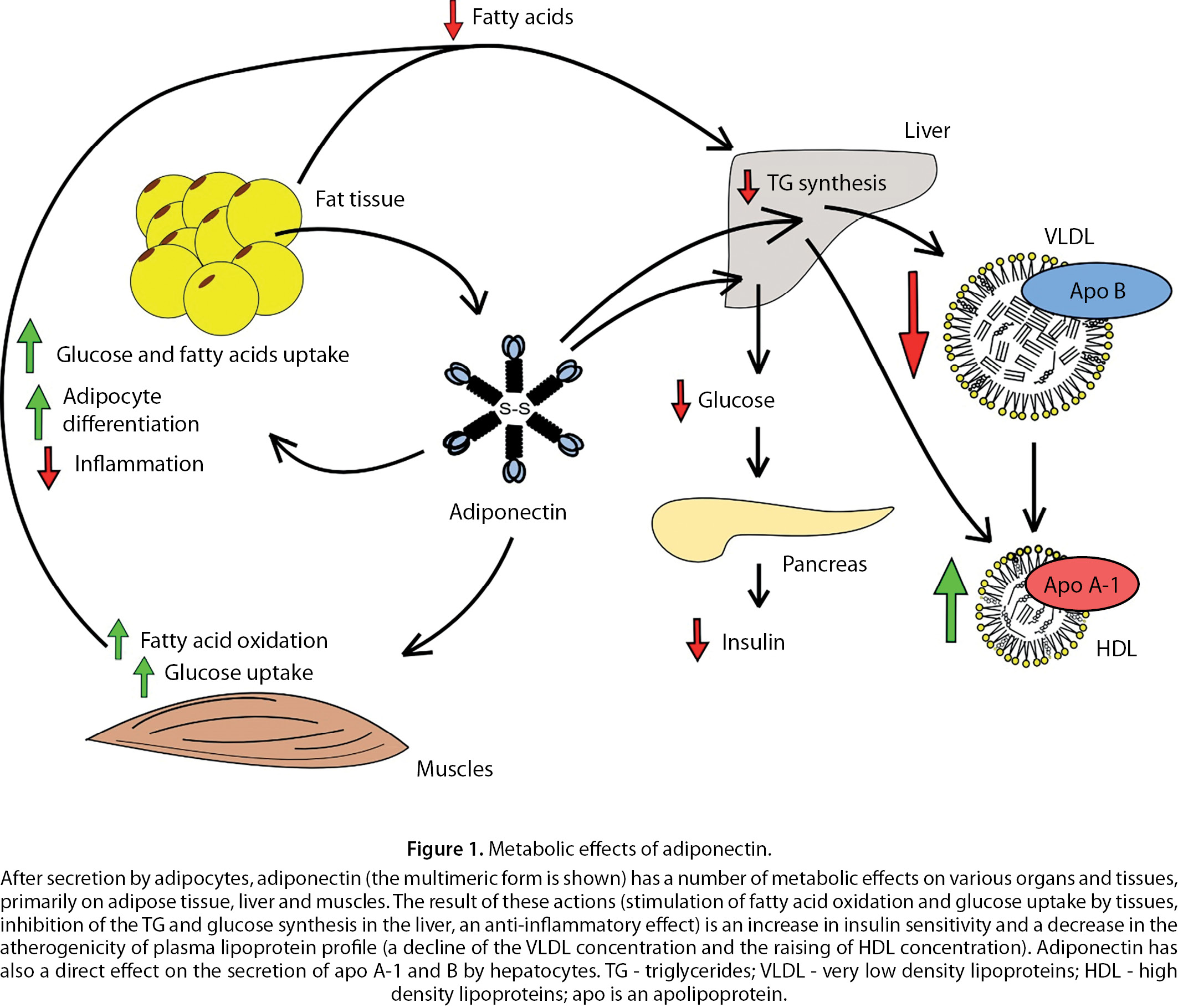
According to others, adiponectin concentration was not an independent determinant of LP levels in plasma [36]. Perhaps, such contradictions are explained mainly by the indirect nature of the relationship between the adiponectin level and the spectrum of plasma LP.
Overexpression of the adiponectin gene in animals, as well as the introduction of recombinant adiponectin to them, prevented the development of IR induced by a high-fat diet, and also caused a decline in the content of FA and TG in plasma [5][22][28][37][38]. Knockout of the adiponectin gene, on the contrary, led to the development of moderate IR, increasing in the level of fatty acids and hypertriglyceridemia [26][39][40].
And finally, mutations in the adiponectin gene, which prevent its multimerization, lead to a decrease in the content of this adipokine in the blood, especially its high-molecular forms, and to the early development of obesity and metabolic syndrome in such individuals [10][11]. There is an evidence that in patients with some mutations in this gene the expression of adiponectin in adipose tissue is increasing, while its receptor - decreasing [11].
In addition to the above pathways, adiponectin is able to affect metabolic processes through its cytokine-like action. Thus, overexpression of the adiponectin gene in adipose tissue in obese mice inhibited the mononuclear cells infiltration of adipose tissue and local production of proinflammatory cytokines [18]. Suppression of the inflammatory process in adipose tissue by adiponectin can mitigate IR and dyslipidemia induced by the action of proinflammatory cytokines [41].
SIGNAL PATHWAYS OF ADIPONECTIN
Adiponectin acts through specific receptors of two types: AdipoR1 and AdipoR2. These receptors are present in almost all cell types and tissues; the highest expression of AdipoR1 was found in cardiac and skeletal muscles, liver, leukocytes, brain, lungs, and AdipoR2 - in muscles, liver, and lungs [3]. AdipoR1 and AdipoR2 are transmembrane proteins, each containing 7 transmembrane domains and having a homology of 66.7%. Their N-ends are turned inside the cell, while the C-ends - outward that oppositely reflects the topology of G-protein-coupled receptors [3]. AdipoRs belong to the family of progesterone and adiponectin receptors (PAQRs) [42]. The homology of AdipoRs with G-protein-coupled receptors is very low [3].
According to X-ray diffraction analysis, a zinc binding site was found in the transmembrane region of AdipoR1, coordinated by three residues of histidine II and VII of the transmembrane helices and aspartate III of the helix [43]. The zinc-binding motif is involved in adiponectin activation of AMPK (AMP-activated protein kinase) and PPAR-α (peroxisome proliferator-activated receptors alpha) (see below). A similar zinc binding site is present in a membrane type of alkaline ceramidase, which also includes 7 transmembrane helical regions and forms a zinc catalytic center responsible for ceramidase activity [44]. Although a functional relationship between the activities of adiponectin receptors and ceramidase has been demonstrated [42, 45], evidence that adiponectin receptors possess such activity themselves has not yet been found [46].
AdipoR1 binds with high affinity to oligomeric and globular adiponectin, while AdipoR2 interacts with moderate affinity with all molecular forms of adiponectin [3]. The same authors showed that knockout of the AdipoR1 or AdipoR2 gene, or knockout of both genes in mice led to a decrease in insulin sensitivity and to an increase in the TG content in liver [47].
It should be noted that the expression of adiponectin receptors in obesity is reduced, which, along with a decrease in the concentration of adiponectin in the blood, leads to a weakening of the regulatory effects of this protein on various tissues [48].
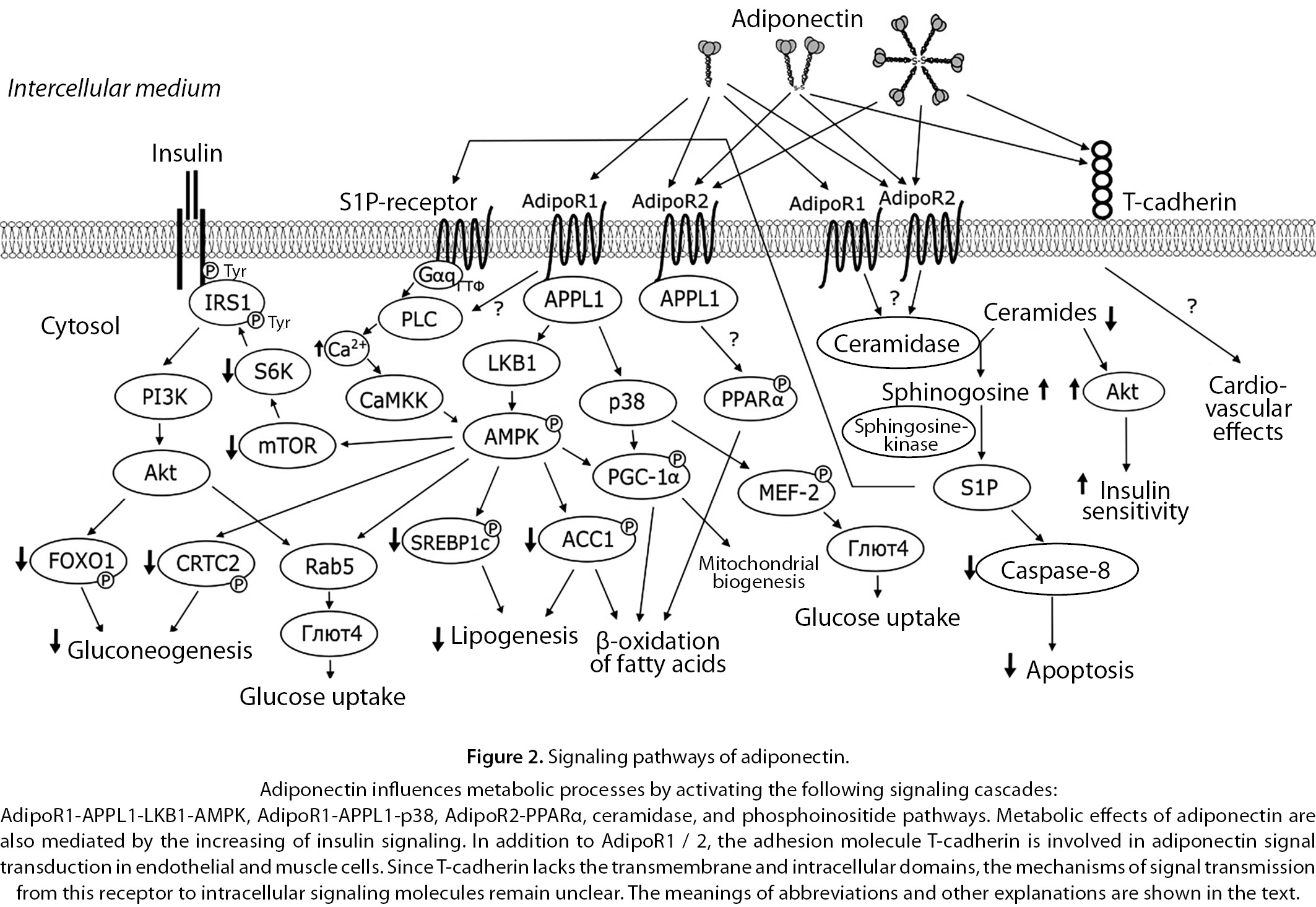
The signal transduction from both adiponectin receptors to intracellular signaling pathways is carried out using the adapter protein APPL1 (an adapter protein containing a phosphotyrosine-binding domain and a leucine zipper sequence 1) [49].
APPL1 is a highly hydrophilic protein lack of transmembrane domains, but containing several structural and functional motifs. With its C-terminal domain, APPL1 directly binds to the intracellular domains of AdipoR2 and AdipoR1 and, after the interaction of the latter with adiponectin, transmits a signal by changing the conformation without phosphorylation of the receptor or adapter protein. Thus, after the interaction of adiponectin with AdipoR1, the associated APPL1, contacting directly, activates protein phosphatase 2A (PP2A) and inhibits protein kinase Cξ (PKCξ), which leads to dephosphorylation of liver kinase B1 (LKB1) and its translocation from the nucleus to the cytoplasm [50].
In the cytoplasm, LKB1 phosphorylates AMPK at position Thr-172, which leads to the activation of the enzyme [51]. AMPK is the most important kinase that regulates energy metabolism in the cell. With a decrease in the concentration of ATP and an increase in AMP and ADP, AMPK is activated restoring the energy balance of the cell, by the stimulation of catabolic processes that generate ATP (capture and oxidation of fatty acids and glucose) and suppression of anabolic processes (synthesis of protein, fatty acids, cholesterol and glycogen, gluconeogenesis) consuming ATP [52]. Activated AMPK is also providing most of the described effects of adiponectin. Thus, AMPK catalyzes the phosphorylation of acetyl-CoA carboxylase 1 (ACC1), a key enzyme of fatty acids synthesis, leading to a decrease in its activity. Since the product of the ACC-catalyzed reaction, malonyl-CoA, is an inhibitor of type 1 carnitine palmitoyltransferase, which ensures the transport of FA into mitochondria, the rate of β-oxidation increases at the suppression of ACC1 activity. Thus, stimulation of AMPK activity, on the one hand, can lead to a decrease in the synthesis of FA and TG in the cell, and, on the other hand, to an increase in the rate of FA oxidation [52].
In addition, AMPK regulates these processes at the genetic level by phosphorylation the transcriptional regulators SREBP-1c (sterol regulatory element-binding protein 1) and PGC-1α (coactivator of the transcription factor PPARγ-1α) [53][54]. Activation of the latter by adiponectin leads to an increase in mitochondrial biogenesis and oxidative metabolism in muscle cells.
After the interaction of adiponectin with AdipoR1, the adapter APPL1 also stimulates MAPK (mitogen-activated protein kinase) p38 by the action of kinases TAK (kinase activated by transforming growth factor beta) and MKK3 (kinase MAPK-3) [55]. Like AMPK, kinase p38 mediates a number of biological effects of adiponectin (influence on mitochondrial biogenesis in muscles, glucose uptake, etc.) [40].
In addition, adiponectin can activate the utilization of energy-rich substrates in an AMPK-independent pathway. In this case, adiponectin interacts with AdipoR2, which transmits signal to PPARα, the activator of transcription of genes, coding the enzymes of peroxisomal and mitochondrial fatty acid oxidation, as well as an activator of a protein that uncouples oxidation and phosphorylation [3][56]. The mechanisms of signaling from AdipoR2 to PPARα remain unknown. As in the case of AdipoR1, the adapter APPL1 is most likely involved in this process [49].
In mammalian cells, there is another adapter protein, APPL2, which is an isoform of APPL1; the homology between them is 45%. APPL2, like APPL1, has several functional and structural domains. It has been shown that APPL2 negatively modulates adiponectin signaling in skeletal muscle. APPL2 binds directly to AdipoR1 or AdipoR2, thus preventing APPL1 from binding to receptors, competitively blocking adiponectin signaling through both of these receptors. In addition, APPL2 forms heterodimers with APPL1, reducing the binding of the latter to AdipoRs and blocking the action of adiponectin. In this case, adiponectin, as well as an insulin, are capable of causing dissociation of APPL1 / APPL2 heterodimers. The role of APPL2 in the regulation of adiponectin signaling has not been fully elucidated [57].
As stated earlier, adiponectin increases the sensitivity of cells to insulin. In skeletal muscle, adiponectin induces tyrosine phosphorylation of the insulin receptor substrate 1 (IRS1) and subsequent activation of Akt kinase, inhibiting p70 S6K kinase phosphorylation and serine phosphorylation in IRS1, thus facilitating insulin signaling. In addition, adiponectin-activated AMPK inhibits mTOR (mammalian target of rapamycin). Further, there is a decrease in the activity of the target of mTOR, p70 S6 kinase, which suppresses the activity of IRS1 by phosphorylation at serine residues [58].
In addition, adiponectin increases insulin sensitivity by activating autophagy in muscle cells that occurs as a consequence of ER and oxidative stress associated with chronic hyperglycemia and high fat load [38][59]. A possible mechanism for participation of APPL1 in increasing the insulin sensitivity has also been described; APPL facilitates the interaction of the insulin receptor with IRS1 [60].
In addition to increasing insulin sensitivity, adiponectin stimulates glucose uptake by skeletal myocytes and adipocytes using several signaling pathways: AMPK [17][49][61], p38 kinase [49][62], and Akt [49]. They all lead to translocation of GLUT4 to the cell membrane by activating the GTPase Rab5 and / or stimulating the expression of the SLC2A4 gene encoding GLUT4 through the activation of the transcriptional regulator MEF-2.
Suppression of hepatic glucose production by adiponectin occurs in several ways. First, AMPK, activated by LKB1, phosphorylates CRTC2, the CREB (cAMP response element binding protein) transcriptional coactivator, inducing the transcription of gluconeogenesis genes, PEPCK (phosphoenolpyruvate carboxykinase) and G6PC (glucose-6-phosphatase) 63]. Phosphorylation of CRTC2 leads to blocking of the translocation of this factor into the nucleus, as a result of which the transcriptional activity of CREB is decreasing [63][64]. In addition, the enhancing of insulin sensitivity also contributes to suppression of gluconeogenesis, in particular through Akt-dependent phosphorylation and degradation of FOXO1 (forkhead box protein O1), another transcriptional activator of gluconeogenesis genes [64].
Another possible pathway for signal transmission from adiponectin receptors is the stimulation of ceramidase activity. As noted earlier, AdipoRs are likely to possess such activity, although there is no direct evidence for this [42][45][46]. Activation of AdipoR1 and AdipoR2 in various tissues (liver, adipose tissue, cardiomyocytes, pancreatic β-cells) leads to a decrease in the concentration of ceramides in them and an increase in the level of their deacylation product, sphingosine. The latter then undergoes phosphorylation to form a biologically active product - sphingosine-1-phosphate (S1P) [42][45]. In turn, S1P activates G-protein-coupled S1P receptors, some of which transmit a signal to the cell via the phosphoinositide mechanism [65]. One of the consequences of S1P receptors activation can be the up-regulation of AMPK by the release of Ca2+ ions into the cytosol, followed by the activation of CaMKK kinase [42].
On the other hand, since the accumulation of ceramides in adipose tissue, muscles and liver leads to suppression of the insulin signal due to inhibition of Akt kinase [66], a decrease in the concentration of ceramides upon activation of AdipoRs is one of the possible mechanisms of increasing insulin sensitivity by adiponectin [46]. An indirect confirmation of this is an enhancement of insulin sensitivity upon the increase in ceramidase activity in the liver and adipose tissue in mice with overexpression of adiponectin receptors [45].
In addition to adiponectin receptors AdipoR1 / 2, some cells, primarily endothelial and muscle cells, interact with adiponectin via the T-cadherin adhesion protein [12].
On the cell membrane, T-cadherin is attached by a glycosylphosphatidylinositol anchor [12]. Probably, lipid rafts of the cytoplasmic membrane are involved in signal transduction from this receptor [67]. T-cadherin interacts with hexameric and multimeric adiponectin, but not with its trimeric forms [12]. T-cadherin sites involved in the binding of adiponectin have been established: of 5 extracellular T-cadherin repeats (EC), those are the domains EC1 and EC2 located at the end of the receptor, which are also responsible for intercellular cells adhesion [68]. According to Denzel et al. (2010) [69] and Matsuda et al. (2015) [70], T-cadherin serves for the accumulation of adiponectin in tissues, in which this adipokine is not synthesized or is synthesized in small quantities (muscles, heart, and aorta).
Knockout of gene encoding T-cadherin in mice eliminates the beneficial effects of adiponectin on tissue revascularization after ischemia [13][69] and on atherogenesis in apoE-deficient mice [71].
In addition to adiponectin, T-cadherin also binds a number of other ligands, one of which are low-density lipoproteins (LDL) [67]. The latter, due to the indicated interaction, is leading to mobilization of intracellular Ca2+, triggering the migration and proliferation of vascular smooth myocytes in vitro. Adiponectin suppresses Ca2+ signaling of LDL, competing with the latter for binding to T-cadherin [67]. These data are in a good agreement with ability of adiponectin to bind plasma LDL, leading to a change in interactions of these parters with cells [72]. The relevance of data cited on the physiology of LDL and adiponectin, as well as on the mechanisms of signal transduction from T-cadherin to intracellular targets, remain unclear.
The described signaling pathways of adiponectin are schematically shown in Fig. 2.
CONCLUSION
Available data indicate a pronounced uniqueness of adiponectin as a hormone (signaling molecule). It has primarily a high (3–6 orders of magnitude higher than other hormones) and almost constant concentration in the blood. If we add to this the presence of several molecular forms in adiponectin with different affinities for several types of receptors with different signaling chains, then the origin of contradictions and even some inexplicability of the described adiponectin effects become clear.
In addition, the regulation of adiponectin synthesis and, in particular, the formation of its molecular forms remains poorly understood.
It can be assumed that the effect of adiponectin depends not so much on its concentration in blood, but on the reaction of cells to this protein, i.e. on the presence of one or another type of receptor (which, perhaps, not all of them are known yet), on the presence, concentration, and activity of one or another type of adapter protein competing with each other, as well as on the «availability» of signal cascades induced by other signaling molecules. As an evidence for this hypothesis, we can provide data on the dependency of the adiponectin effects not only on the inactivation (knockout or knockdown) or activation (overexpression) of adiponectin receptors, but also on the activity of adapter proteins [73]. It should not be overlooked that one of the main pathways of adiponectin signal transmission is carried out using AMPK, which is inhibited by ATP; therefore, the energy balance of the cell can have a significant modulating impact on the effects of adiponectin.
Obviously, not all signal cascades of adiponectin have been discovered. The mechanisms of adiponectin signaling via AdipoR2 and also via T-cadherin still require the investigations. In addition, the studies of signaling mechanisms of the individual adiponectin molecular forms action are extremely rare. All above indicates a complex nature of adiponectin signaling, many of the mechanisms of which remain unrevealed, and, possibly, the near future will bring us significant progress in this area.
ADDITIONAL INFORMATION
Funding sources. The work was supported by the state assignment, project number: 0557-2019-0011.
Conflict of interest. The authors declare no obvious and potential conflicts of interest related to the content of this article.
Contribution of authors. D.A. Tanyansky - literature search, analysis, writing and editing the manuscript; A.D. Denisenko - literature search, analysis, writing and editing the manuscript.
All of the authors approved the final version of the article before publication, agreed to be responsible for all aspects of the work, implying proper examination and resolution of issues relating to the accuracy or integrity of any part of the work.
References
1. Sethi J K, Vidal-Puig AJ. Adipose tissue function and plasticity orchestrate nutritional adaptation. J Lipid Res. 2007;48:1253-1262. doi: https://doi.org/10.1194/jlr.R700005-JLR200
2. Scherer PE, Williams S, Fogliano M, et al. A novel serum protein similar to C1q, produced exclusively in adipocytes. J Biol Chem. 1995;270:26746-26749. doi: https://doi.org/10.1074/jbc.270.45.26746
3. Yamauchi T, Kamon J, Ito Y, et al. Cloning of adiponectin receptors that mediate antidiabetic metabolic effects. Nature. 2003;423:762-769. doi: https://doi.org/10.1038/nature01705
4. Tsao TS. Assembly of adiponectin oligomers. Rev Endocr Metab Disord. 2014;15(2):125-136. doi: https://doi.org/10.1007/s11154-013-9256-6
5. Fruebis J, Tsao T-S, Javorschi S, et al. Proteolytic cleavage product of 30-kDa adipocyte complement-related protein increases fatty acid oxidation in muscle and causes weight loss in mice. Proc Natl Acad Sci USA. 2001;98:2005-2010. doi: https://doi.org/10.1073/pnas.041591798
6. Waki H, Yamauchi T, Kamon J, et al. Generation of globular fragment of adiponectin by leucocyte elastase secreted by monocytic cell line THP-1. Endocrinology. 2005;146:790-796. doi: https://doi.org/10.1210/en.2004-1096
7. Wang ZV, Scherer PE. DsbA-L is a versatile player in adiponectin secretion. Proc Natl Acad Sci U S A. 2008;105(47):18077-18078. doi: https://doi.org/10.1073/pnas.0810027105
8. Liu M, Xiang R, Wilk SA, et al. Fat-specific DsbA-L overexpression promotes adiponectin multimerization and protects mice from diet-induced obesity and insulin resistance. Diabetes. 2012;61(11):2776-2786. doi: https://doi.org/10.2337/db12-0169
9. Pajvani U, Du X, Combs T, et al. Structure-function studies of the adipocyte-secreted hormone Acrp30/adiponectin. J Biol Chem. 2003;278:9073-9085. doi: https://doi.org/10.1074/jbc.M207198200
10. Waki H, Yamauchi T, Kamon J, et al. Impaired multimerization of human adiponectin mutants associated with diabetes. Molecular structure and multimer formation of adiponectin. J Biol Chem. 2003;278(41):40352-40363. doi: https://doi.org/10.1074/jbc.M300365200.
11. Bueno AC, Sun K, Martins CS, et al. Antonini SR. A novel ADIPOQ mutation (p.M40K) impairs assembly of high-molecular-weight adiponectin and is associated with early-onset obesity and metabolic syndrome. J Clin Endocrinol Metab. 2014;99:E683-E693. doi: https://doi.org/10.1210/jc.2013-3009
12. Hug C, Wang J, Ahmad NS, et al. T-cadherin is a receptor for hexameric and high-molecular-weight forms of Acrp30/adiponectin. Proc Natl Acad Sci USA. 2004;101(28):10308-10313. doi: https://doi.org/10.1073/pnas.0403382101
13. Parker-Duffen J, Nakamura K, Silver M, et al. T-cadherin is essential for adiponectin-mediated revascularization. J Biol Chem. 2013;288:24886-24897. doi: https://doi.org/10.1074/jbc.M113.454835
14. Wanninger J, Liebisch G, Eisinger K, et al. Adiponectin isoforms differentially affect gene expression and the lipidome of primary human hepatocytes. Metabolites. 2014;4(2):394-407. doi: https://doi.org/10.3390/metabo4020394.
15. He Y, Lu L, Wei X, et al. The multimerization and secretion of adiponectin are regulated by TNF-alpha. Endocrine. 2016;51:456-468. doi: https://doi.org/10.1007/s12020-015-0741-4
16. Razgildina ND, Brovin DL, Pobozheva IA, et al. Adiponectine gene expression in subcutaneous and intra-abdominal adipose tissue in women with varying degrees of obesity. Tsitologiya. 2018;60:531-535. (In Russ.). doi: https://doi.org/10.31116/tsitol.2018.07.08
17. Fu Y, Luo N, Klein RL, Garvey WT. Adiponectin promotes adipocyte differentiation, insulin sensitivity, and lipid accumulation. J Lipid Res. 2005;46(7):1369-1379. doi: https://doi.org/10.1194/jlr.M400373-JLR200
18. Kim JY, van de Wall E, Laplante M, et al. Obesity-associated improvements in metabolic profile through expansion of adipose tissue. J Clin Invest. 2007;117:2621-2637. doi: https://doi.org/10.1172/JCI31021
19. Wu X, Motoshima H, Mahadev K, et al. Involvement of AMP-activated protein kinase in glucose uptake stimulated by the globular domain of adiponectin in primary rat adipocytes. Diabetes. 2003;52:1355-1363. doi: https://doi.org/10.2337/diabetes.52.6.1355
20. Qiao L, Kinney B, Schaack J, Shao J. Adiponectin inhibits lipolysis in mouse adipocytes. Diabetes. 2011;60(5):1519-1527. doi: https://doi.org/10.2337/db10-1017
21. Wedellova Z, Kovacova Z, Tencerova M, et al. The impact of full-length, trimeric and globular adiponectin on lipolysis in subcutaneous and visceral adipocytes of obese and non-obese women. PLoS One. 2013;8(6):e66783. doi: https://doi.org/10.1371/journal.pone.0066783
22. Qiao L, Zou C, van der Westhuyzen DR, Shao J. Adiponectin reduces plasma triglyceride by increasing VLDL triglyceride catabolism. Diabetes. 2008;57:1824-1833. doi: https://doi.org/10.2337/db07-0435
23. Yamauchi T, Kamon J, Waki H, et al. The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat Med. 2001;7(8):941-946. doi: https://doi.org/10.1038/90984
24. Bruce CR, Mertz VA, Heigenhauser GJF, Dyck DJ. The stimulatory effect of globular adiponectin on insulin-stimulated glucose uptake and fatty acid oxidation is impaired in skeletal muscle from obese subjects. Diabetes. 2005;54:3154-3160. doi: https://doi.org/10.2337/diabetes.54.11.3154
25. Ritchie IR, Dyck DJ. Rapid loss of adiponectin-stimulated fatty acid oxidation in skeletal muscle of rats fed a high fat diet is not due to altered muscle redox state. PLoS One. 2012;7(12):e52193. doi: https://doi.org/10.1371/journal.pone.0052193
26. Maeda N, Shimomura I, Kishida K, et al. Diet-induced insulin resistance in mice lacking adiponectin/ACRP30. Nat Med. 2002;8(7):731-737. doi: https://doi.org/10.1038/nm724
27. Jung TW, Choi HY, Lee SY, et al. Salsalate and adiponectin improve palmitate-induced insulin resistance via inhibition of selenoprotein P through the AMPK-FOXO1α pathway. PLoS One. 2013;8(6):e66529. doi: https://doi.org/10.1371/journal.pone.0066529
28. Miller RA, Chu Q, Le Lay J, et al. Adiponectin suppresses gluconeogenic gene expression in mouse hepatocytes independent of LKB1-AMPK signaling. J Clin Invest. 2011;121:2518-2528. doi: https://doi.org/10.1172/JCI45942
29. Tschritter O, Fritsche A, Thamer C, et al. Plasma adiponectin concentrations predict insulin sensitivity of both glucose and lipid metabolism. Diabetes. 2003;52(2):239-243. doi: https://doi.org/10.2337/diabetes.52.2.239
30. Lara-Castro C, Luo N, Wallace P, et al. Adiponectin multimeric complexes and the metabolic syndrome trait cluster. Diabetes. 2006;55:249-259. doi: https://doi.org/10.2337/diabetes.55.01.06.db05-1105
31. Tanyanskiy DA, Firova EM, Shatilina LV, Denisenko AD. Role of adipokines and nonesterified fatty acids in the development of insulin resistance. Problems of Endocrinology. 2009;55(3):13-16. (In Russ.). doi: https://doi.org/10.14341/probl200955313-16
32. Hotta K, Funahashi T, Arita Y, et al. Plasma concentrations of a novel, adipose-specific protein, adiponectin, in type 2 diabetic patients. Arterioscler Thromb Vasc Biol. 2000;20:1595-1599. doi: https://doi.org/10.1161/01.atv.20.6.1595
33. Abbasi F, Chu J, Lamendola C, et al. Discrimination between obesity and insulin resistance in the relationship with adiponectin. Diabetes. 2004;53:585-590. doi: https://doi.org/10.2337/diabetes.53.3.585
34. Fisher EA, Feig JE, Hewing B, et al. High-density lipoprotein function, dysfunction, and reverse cholesterol transport. Arterioscler Thromb Vasc Biol. 2012;32(12):2813-2820. doi: https://doi.org/10.1161/ATVBAHA.112.300133
35. Matsuura F, Oku H, Koseki M, et al. Adiponectin accelerates reverse cholesterol transport by increasing high density lipoprotein assembly in the liver. Biochem Biophys Res Commun. 2007;358:1091-1095. doi: https://doi.org/10.1016/j.bbrc.2007.05.040
36. Salas-Salvadó J, Granada M, Bulló M, et al. Plasma adiponectin distribution in a Mediterranean population and its association with cardiovascular risk factors and metabolic syndrome. Metabolism. 2007;56(11):1486‐1492. doi: https://doi.org/10.1016/j.metabol.2007.06.014
37. Ma Y, Liu D. Hydrodynamic delivery of adiponectin and adiponectin receptor 2 gene blocks high-fat diet-induced obesity and insulin resistance. Gene Ther. 2013;20(8):846-852. doi: https://doi.org/10.1038/gt.2013.8
38. Liu Y, Palanivel R, Rai E, et al. Adiponectin stimulates autophagy and reduces oxidative stress to enhance insulin sensitivity during high-fat diet feeding in mice. Diabetes. 2015;64(1):36-48. doi: https://doi.org/10.2337/db14-0267
39. Nawrocki AR, Rajala MW, Tomas E, et al. Mice lacking adiponectin show decreased hepatic insulin sensitivity and reduced responsiveness to peroxisome proliferator-activated receptor gamma agonists. J Biol Chem. 2006;281(5):2654-2660. doi: https://doi.org/10.1074/jbc.M505311200
40. Qiao L, Kinney B, Yoo HS, et al. Adiponectin increases skeletal muscle mitochondrial biogenesis by suppressing mitogen-activated protein kinase phosphatase-1. Diabetes. 2012;61(6):1463-1470. doi: https://doi.org/10.2337/db11-1475
41. Urschel K, Cicha I. TNF-α in the cardiovascular system: from physiology to therapy. International Journal of Interferon, Cytokine and Mediator Research. 2015;7:9-25. doi: https://doi.org/10.2147/IJICMR.S64894
42. Holland WL, Miller RA, Wang ZV, et al. Receptor-mediated activation of ceramidase activity initiates the pleiotropic actions of adiponectin. Nat Med. 2011;17(1):55-63. doi: https://doi.org/10.1038/nm.2277
43. Tanabe H, Fujii Y, Okada-Iwabu M, et al. Crystal structures of the human adiponectin receptors. Nature. 2015;520(7547):312-316. doi: https://doi.org/10.1038/nature14301
44. Vasiliauskaité-Brooks I, Healey RD, Rochaix P, et al. Structure of a human intramembrane ceramidase explains enzymatic dysfunction found in leukodystrophy. Nat Commun. 2018;9(1):5437. doi: https://doi.org/10.1038/s41467-018-07864-w
45. Holland WL, Xia JY, Johnson JA, et al. Inducible overexpression of adiponectin receptors highlight the roles of adiponectin-induced ceramidase signaling in lipid and glucose homeostasis. Mol Metab. 2017;6(3):267-275. doi: https://doi.org/10.1016/j.molmet.2017.01.002
46. Straub LG, Scherer PE. Metabolic Messengers: Adiponectin. Nat Metab. 2019;1(3):334-339. doi: https://doi.org/10.1038/s42255-019-0041-z
47. Yamauchi T, Nio Y, Maki T, et al. Targeted disruption of AdipoR1 and AdipoR2 causes abrogation of adiponectin binding and metabolic actions. Nat Med. 2007;13:332-339. doi: https://doi.org/10.1038/nm1557
48. Tsuchida A, Yamauchi Т, Ito Y, et al. Insulin/Foxo1 pathway regulates expression levels of adiponectin receptors and adiponectin sensitivity. J Biol Chem. 2004;279:30817-30822. doi: https://doi.org/10.1074/jbc.M402367200
49. Mao X, Kikani CK, Riojas RA, et al. APPL1 binds to adiponectin receptors and mediates adiponectin signalling and function. Nat Cell Biol. 2006;8(5):516-523. doi: https://doi.org/10.1038/ncb1404
50. Deepa SS, Zhou L, Ryu J, et al. APPL1 mediates adiponectin-induced LKB1 cytosolic localization through the PP2A-PKCz signaling pathway. Mol Endocrinol. 2011;25:1773-1785. doi: https://doi.org/10.1210/me.2011-0082
51. Shaw RJ, Kosmatka M, Bardeesy N, et al. The tumor suppressor LKB1 kinase directly activates AMP-activated kinase and regulates apoptosis in response to energy stress. Proc Natl Acad Sci USA. 2004;101(10):3329-3335. doi: https://doi.org/10.1073/pnas.0308061100
52. Garcia D, Shaw RJ. AMPK: Mechanisms of cellular energy sensing and restoration of metabolic balance. Mol Cell. 2017;66(6):789-800. doi: https://doi.org/10.1016/j.molcel.2017.05.032
53. Li Y, Xu S, Mihaylova MM, et al. AMPK phosphorylates and inhibits SREBP activity to attenuate hepatic steatosis and atherosclerosis in diet-induced insulin-resistant mice. Cell Metab. 2011;13(4):376-388. doi: https://doi.org/10.1016/j.cmet.2011.03.009
54. Iwabu M, Yamauchi T, Okada-Iwabu M, et al. Adiponectin and AdipoR1 regulate PGC-1alpha and mitochondria by Ca(2+) and AMPK/SIRT1. Nature. 2010;464(7293):1313-1319. doi: https://doi.org/10.1038/nature08991
55. Xin X, Zhou L, Reyes CM, et al. APPL1 mediates adiponectin-stimulated p38 MAPK activation by scaffolding the TAK1-MKK3-p38 MAPK pathway. Am J Physiol Endocrinol Metab. 2011;300(1):E103-E110. doi: https://doi.org/10.1152/ajpendo.00427.2010
56. Varga T, Czimmerer Z, Nagy L. PPARs are a unique set of fatty acid regulated transcription factors controlling both lipid metabolism and inflammation. Biochim Biophys Acta. 2011;1812(8):1007-1022. doi: https://doi.org/10.1016/j.bbadis.2011.02.014
57. Wang C, Xin X, Xiang R, et al. Yin-Yang regulation of adiponectin signaling by APPL isoforms in muscle cells. J Biol Chem. 2009;284(46):31608-31615. doi: https://doi.org/10.1074/jbc.M109.010355
58. Wang C, Mao X, Wang L, et al. Adiponectin sensitizes insulin signaling by reducing p70 S6 kinase-mediated serine phosphorylation of IRS-1. J Biol Chem. 2007;282(11):7991-7996. doi: https://doi.org/10.1074/jbc.M700098200
59. Ahlstrom P, Rai E, Chakma S, et al. Adiponectin improves insulin sensitivity via activation of autophagic flux. J Mol Endocrinol. 2017;59(4):339-350. doi: https://doi.org/10.1530/JME-17-0096
60. Ryu J, Galan AK, Xin X, et al. APPL1 potentiates insulin sensitivity by facilitating the binding of IRS1/2 to the insulin receptor. Cell Rep. 2014;7:1227-1238. doi: https://doi.org/10.1016/j.celrep.2014.04.006
61. McGee SL, van Denderen BJ, Howlett KF, et al. AMP-activated protein kinase regulates GLUT4 transcription by phosphorylating histone deacetylase 5. Diabetes. 2008;57(4):860-867. doi: https://doi.org/10.2337/db07-0843
62. Montessuit C, Rosenblatt-Velin N, Papageorgiou I, et al. Regulation of glucose transporter expression in cardiac myocytes: p38 MAPK is a strong inducer of GLUT4. Cardiovasc Res. 2004;64(1):94-104. doi: https://doi.org/10.1016/j.cardiores.2004.06.005
63. Yoon YS, Ryu D, Lee MW, et al. Adiponectin and thiazolidinedione targets CRTC2 to regulate hepatic gluconeogenesis. Exp Mol Med. 2009;41(8):577-583. doi: https://doi.org/10.3858/emm.2009.41.8.063
64. Oh KJ, Han HS, Kim MJ, Koo SH. CREB and FoxO1: two transcription factors for the regulation of hepatic gluconeogenesis. BMB Rep. 2013;46(12):567-574. doi: https://doi.org/10.5483/bmbrep.2013.46.12.248
65. Cannavo A, Liccardo D, Komici K, et al. Sphingosine Kinases and Sphingosine 1-Phosphate Receptors: Signaling and Actions in the Cardiovascular System. Front Pharmacol. 2017;8:9. doi: https://doi.org/10.3389/fphar.2017.00556
66. Chavez JA, Summers SA. A ceramide-centric view of insulin resistance. Cell Metab. 2012;15(5):585-594. doi: https://doi.org/10.1016/j.cmet.2012.04.002
67. Balatskaya MN, Balatskii AV, Sharonov GV, et al. T-cadherin as a novel receptor regulating metabolism in the blood vessel and heart cells: from structure to function. J Evol Biochem Phys. 2016;52:103-118. (In Russ.). doi: https://doi.org/10.1134/S0022093016020010
68. Fukuda S, Kita S, Obata Y, et al. The unique prodomain of T-cadherin plays a key role in adiponectin binding with the essential extracellular cadherin repeats 1 and 2. J Biol Chem. 2017;292(19):7840-7849. doi: https://doi.org/10.1074/jbc.M117.780734
69. Denzel MS, Scimia MC, Zumstein PM, et al. T-cadherin is critical for adiponectin-mediated cardioprotection in mice. J Clin Invest. 2010;120(12):4342-4352. doi: https://doi.org/10.1172/JCI43464
70. Matsuda K, Fujishima Y, Maeda N, et al. Positive feedback regulation between adiponectin and T-cadherin impacts adiponectin levels in tissue and plasma of male mice. Endocrinology. 2015;156(3):934-946. doi: https://doi.org/10.1210/en.2014-1618
71. Fujishima Y, Maeda N, Matsuda K, et al. Adiponectin association with T‐cadherin protects against neointima proliferation and atherosclerosis. FASEB J. 2017;31(4):1571-1583. doi: https://doi.org/10.1096/fj.201601064R
72. Kakino A, Fujita Y, Ke L-Y, et al. Adiponectin forms a complex with atherogenic LDL and inhibits its downstream effects. J Lipid Res. 2021;62:100001. doi: https://doi.org/10.1194/jlr.RA120000767
73. Cheng KK, Iglesias MA, Lam KS, et al. APPL1 potentiates insulin-mediated inhibition of hepatic glucose production and alleviates diabetes via Akt activation in mice. Cell Metab. 2009;9:417-427. doi: https://doi.org/10.1016/j.cmet.2009.03.01
About the Authors
D. A. TanyanskiyRussian Federation
Dmitry A. Tanyanskiy, MD, PhD; Researcher ID: G-3307-2015; Scopus Author ID: 53878682400; eLibrary SPIN: 9303-9445;
12 acad. Pavlov street, 197376 Saint-Petersburg
Competing Interests:
not
A. D. Denisenko
Russian Federation
Alexander D. Denisenko, MD, PhD, Professor; Researcher ID: G-4774-2015; Scopus Author ID: 7005191805; eLibrary SPIN: 7496-1449.
Saint-Petersburg
Competing Interests:
not
Supplementary files
|
|
1. Figure 1. Metabolic effects of adiponectin. | |
| Subject | ||
| Type | Исследовательские инструменты | |
View
(673KB)
|
Indexing metadata ▾ | |
|
|
2. Figure 2. Signaling pathways of adiponectin. | |
| Subject | ||
| Type | Исследовательские инструменты | |
View
(905KB)
|
Indexing metadata ▾ | |
Review
For citations:
Tanyanskiy D.A., Denisenko A.D. The influence of adiponectin on carbohydrates, lipids, and lipoproteins metabolism: analysis of signaling mechanisms. Obesity and metabolism. 2021;18(2):103-111. https://doi.org/10.14341/omet12754

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND 4.0).










































